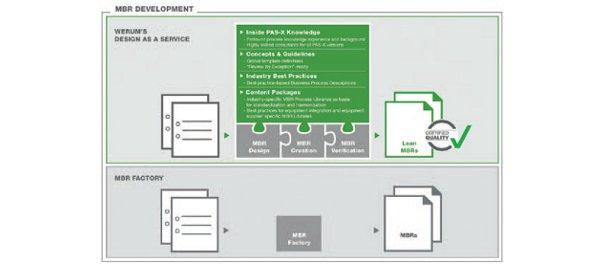
“Design as a Service” offers the customer-specific creation of MBRs and the efficient operation of Werum’s PAS-X manufacturing execution system.
Development of new drugs, process harmonization and standardization or efficient operation of manufacturing execution systems (MES) – pharmaceutical and biopharmaceutical companies face major challenges to remain competitive. In this regard, electronic master batch records (MBRs) are an essential factor. Werum
IT Solutions now offers its customers a new service: “Design as a Service” – a complete solution for the cost-effective creation of lean, high-quality and streamlined MBRs. The package comprises pre-configured PAS-X Content Packages with industry-specific content based on best practices, ready-to-use concepts and guidelines for the efficient creation and optimization of MBRs and profound, first-hand PAS-X knowledge. As the company who developed PAS-X, Werum is able to provide an “end-to-end” MES solution that meets all customer requirements in terms of software, delivery and services. Through “Design as a Service”, Werum does not only support the cost-effective high-quality creation of MBRs for new MES installations but also supports customers who already operate an MES and rather focus on process harmonization, PAS-X upgrades or the MBR creation for new products (ad-hoc service). As PAS-X is Werum’s proprietary software product, Werum can also consider future PAS-X functions when creating MBRs. “Design as a Service” addresses all phases of MBR development, such as MBR specification, MBR creation and finally MBR verification. The resulting MBRs are lean, efficient and optimized to meet the customer requirements in regard to “Review by Exception”.
“Our ‘Design as a Service’ offering allows us to provide our pharmaceutical and biopharmaceutical customers with much more than the mass conversion of paper-based recipes into digital copies which is usually referred to as MBR factory,” says Martin Gent, Consultant, Werum IT Solutions GmbH. “Our approach starts at an earlier stage and we reflect about the manufacturing processes together with our customers. Even during the MBR specification phase we consider the entire spectrum of manufacturing procedures, system environment and review processes. In combination with the profound process know-how of our staff we ensure optimized high-quality MBRs from the very beginning – an important prerequisite for the efficient operation of our PAS-X MES. Customers who want to save costs by commissioning the mere creation of MBRs often face extra costs when they need to revise their MBRs.”
Werum IT Solutions is the world’s leading supplier of manufacturing execution systems (MES) and manufacturing IT solutions for the pharmaceutical and biopharmaceutical industries. Its out-of-the-box PAS-X software product is run by 17 of the world’s top 30 pharmaceutical and biotech companies but also by many mid-sized manufacturers. Werum’s manufacturing IT solutions help pharma manufacturers to increase efficiency, improve productivity, and meet regulatory requirements. Founded in 1969, the IT company employs about 420 people at its headquarters in Lüneburg, Germany, and at ten other locations in Europe, America and Asia. Werum is part of Medipak Systems, the Pharma Systems business area of the international Körber technology group. Körber unites worldwide nearly 12,000 professionals in industry-leading companies, achieving annual earnings of more than 2.3 billion Euros. As a Medipak Systems company, Werum provides integrated IT solutions for all phases of pharmaceutical and biopharmaceutical production – including process development, commercial production, and packaging as well as track & trace serialization.